What is Sulphuric Acid

Sulphuric acid is a colourless, odourless and a highly corrosive mineral acid. It is highly acidic in nature and is also termed hygroscopic. Sulphuric acid is an important commodity chemical and used in a various industries. There are different concentrations of sulphuric acid available in the market and they are named distilled sulphuric acid, battery acid, chamber acid, fertilizer acid, tower acid, Glover acid and concentrated sulphuric acid. It is widely used in mining industry in the manufacture of zinc and copper and for cleaning metal surfaces. It is used in manufacture of many other chemicals like hydrofluoric acid. As of 2017, the world’s total sulphuric acid protections stands at 270 million tonnes and is expected to increase.

Manufacturing Process
Pure sulphuric acid is not found in nature by default but the diluted sulphuric acid is obtained by acid rain. There are various methods by which sulphuric acid can be manufactured – Contact process, wet sulphuric acid process and other methods like using metabisulfite.
Contact Process
Sulphur is burned to form sulphur dioxide which is then oxidised to form sulphur trioxide using vanadium as catalyst. The sulphur trioxide is mixed with 97-98% sulphuric acid to produce oleum, which on treatment with water gives sulphuric acid.
S (s) + O2 (g) → SO2 (g)
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g) (in presence of V2O5)
H2SO4 (l) + SO3 (g)→ H2S2O7 (l)
H2S2O7 (l) + H2O (l) → 2 H2SO4 (l)
Wet Sulphuric Acid Process
Sulphur is burned to form sulphur dioxide or hydrogen sulphide is burned to form sulphur dioxide. This in turn is treated with oxygen to form sulphur trioxide. Sulphur trioxide is hydrated to form gaseous sulphuric acid, and then condensed to form liquid sulphuric acid.
S(s) + O2(g) → SO2(g) or 2 H2S + 3 O2 → 2 H2O + 2 SO2
2 SO2 + O2 ⇌ 2 SO3
SO3 + H2O → H2SO4(g)
H2SO4(g) → H2SO4(l)

The Uses of Sulphuric Acid
Sulfuric acid is a very important commodity chemical, and indeed, a nation’s sulfuric acid production is a good indicator of its industrial strength. World production in the year 2004 was about 180 million tonnes, with the following geographic distribution: Asia 35%, North America (including Mexico) 24%, Africa 11%, Western Europe 10%, Eastern Europe and Russia 10%, Australia and Oceania 7%, South America 7%.
Detergent Industry
Sulfuric acid is often used as a dehydrating agent when manufacturing dyes, detergents and explosive devices. It effectively draws out condensation and moisture from a diverse range of substances. For example, when sulfuric acid is poured on sugar crystals, the compound removes 11 molecules of water for every molecule of sucrose, leaving a spongy, brittle mass of carbon. The drying properties of sulfuric acid make it corrosive and cause burns on the skin.
Electronic and Automotive Industry
Sulphuric acid is used in lead-acid batteries for cars and other vehicles. Sulphuric acid is usually mixed with water to create chemical reaction that produce electron to make the charge that needed to start vehicle. The electron also circulates through the cathode to allow a safe method for removal of the used electron, because the electron charge cannot be used again by the battery.
Waste Water Treatment Industry
For wastewater treatment, sulphuric acid is used to remove impurities from various substances. Sulphuric acid is used to neutralize the water that contaminate from basic substance and also use to break emulsions. It also used in drinking water treatment to create an improved taste and remove impurities. It also used as raw material in the production of another wastewater treatment compound such as aluminium sulphate.
Other Applications
In oil refinery, sulphuric acid is used as catalyst in production of isooctane. In steel industry, sulphuric acid is used to remove rust and oxidation. Because of its strong acidic properties, sulphuric acid is used as pH neutralization for certain strong basic solution such as sodium hydroxide, calcium carbonate, or lime suspension. The neutralization reaction can rise the temperature. In pulp industry, sulphuric acid is use as pH regulator when recovering pulp chemicals and to produce chlorine dioxide for bleaching.
Hazard Identification
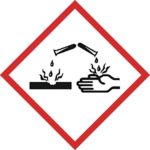

Sulfuric acid is capable of causing very severe burns, especially when it is at high concentrations. In common with other corrosive acids and alkali, it readily decomposes proteinsand lipids through amide and ester hydrolysis upon contact with living tissues, such as skin and flesh. In addition, it exhibits a strong dehydrating property on carbohydrates, liberating extra heat and causing secondary thermal burns. Accordingly, it rapidly attacks the cornea and can induce permanent blindness if splashed onto eyes. If ingested, it damages internal organs irreversibly and may even be fatal. Protective equipment should hence always be used when handling it. Moreover, its strong oxidizing property makes it highly corrosive to many metals and may extend its destruction on other materials. Because of such reasons, damage posed by sulfuric acid is potentially more severe than that by other comparable strong acids, such as hydrochloric acid and nitric acid.
